Small Molecule Drug Development Services
Altasciences understands the challenges involved in developing small molecules from lead candidate identification to approval. Altasciences will work in close collaboration with you to streamline development and accelerate your timeline, fast-tracking your progression from discovery to preclinical and clinical stages of development, and beyond.
HOW WE FAST-TRACK YOUR
SMALL MOLECULE DRUG DEVELOPMENT
When coordinating with multiple CROs for outsourcing, facing delays in timelines can pose unexpected challenges. Our full-service, small molecule drug development solution can conduct certain study activities in parallel. We integrate bioanalytical services, preclinical safety evaluation, formulation development, clinic-ready manufacturing, on-demand clinical pharmacy, and clinical testing up to proof-of-concept, all within one organization, customized to meet your specific needs.

Click each of the drawers below to discover how Altasciences supports your small molecule development from discovery through to Phase IV, and commercialization stages.
DISCOVERY
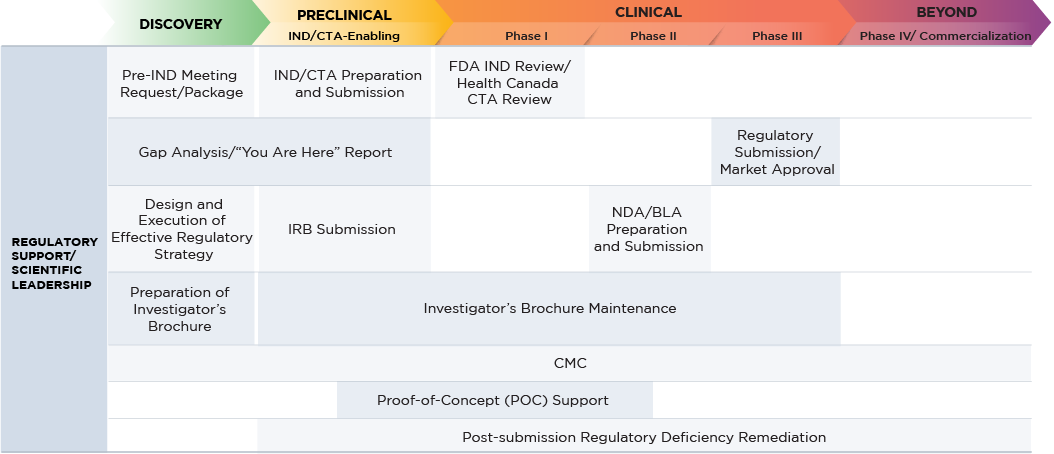
Regulatory Support
- Gap analysis/”you are here” report
- Pre-IND meeting
- Design and implementation of regulatory strategy
Scientific Leadership
- Design and execution of drug development strategy
- Preparation of Investigator’s Brochure (IB)
Bioanalytical Services/Biomarkers
- Discovery and exploratory methods and samples
PRECLINICAL
Regulatory Support
- Gap analysis/”you are here” report
- Pre-IND meeting
- Design and implementation of non-clinical regulatory strategy
- IND/CTA preparation and submission
- IND/CTA meeting
- Investigator's Brochure (IB) preparation and maintenance
Scientific Leadership
- Design and execution of nonclinical strategy
- Preparation of Investigator’s Brochure (IB)
- Post-submission regulatory deficiency remediation (from preclinical through to commercialization)
- Bioanalytical Services
Safety Assessment
- PK/PD studies
- Safety Assessment programs
- Phase I dosage support
- First-in-human (FIH) risk assessment
Manufacturing and Analytical Services
- Drug formulation
Bioanalytical Services/Biomarkers
- Discovery and exploratory methods and samples
Program Management
- Dedicated cross-functional program manager (all phases)
CRO Services
- Organizational
- Scientific
- Trial services (all Phases)
CLINICAL
Regulatory Support
- Gap analysis/”you are here” report
- Design and implementation of clinical strategy
Scientific Leadership
- Design and execution of clinical strategy
- Post-submission regulatory deficiency remediation (from preclinical through Phase IV)
- IB preparation and maintenance (Phase I and II)
Safety Assessment
- PK/PD (Preclinical through Phase I)
- Pre-IND toxicology study packages (Preclinical through Phase I)
Clinical Pharmacology
- FIH studies (Phase I)
- Proof-of-concept (POC) studies (Phase II)
- Customized studies/NDA-enabling (Phase II and III)
Manufacturing and Analytical Services
- GMP clinical and commercial batch manufacturing and analytical testing (from Phase I through Phase IV)
Program Management
- Dedicated cross-functional program manager (all Phases)
CRO Services
- Organizational
- Scientific
- Trial Services (all Phases)
Bioanalytical Services/Biomarkers
- Method development and analysis for clinic sample analysis (Phase I to Phase IV)
BEYOND (PHASE IV/COMMERCIALIZATION)
Scientific Leadership
- Post-submission regulatory deficiency remediation (preclinical through Phase IV)
Manufacturing and Analytical Services
- GMP clinical and commercial batch manufacturing and analytical testing (Phase I through Phase IV)
Program Management
- Dedicated cross-functional program manager (all Phases)
CRO Services
- Organizational
- Scientific
- Trial Services (all Phases)
Bioanalytical Services/Biomarkers
- Regulated sample analysis (Phase I to Phase IV)
SMALL MOLECULE DRUG DEVELOPMENT SERVICES
Altasciences offers an extensive variety of services to bring you through each stage of small molecule development with ease. Explore the sections below to discover how we can reduce your overall timelines by up to 40%, using over 25 years’ experience, and our unique approach to program management.
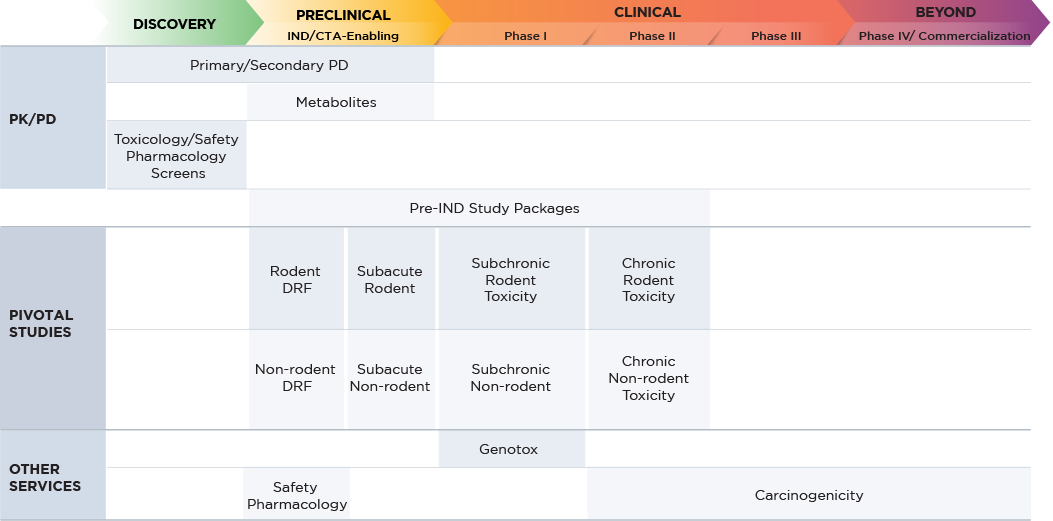
We have a comprehensive offering of in vivo GLP and non-GLP preclinical evaluation studies to thoroughly assess the safety of your molecules. Our solution offering includes IND/CTA- and NDA-enabling toxicology, safety pharmacology, and laboratory services that meet global regulatory requirements.
Fact Sheet: Small Molecule Safety Assessment
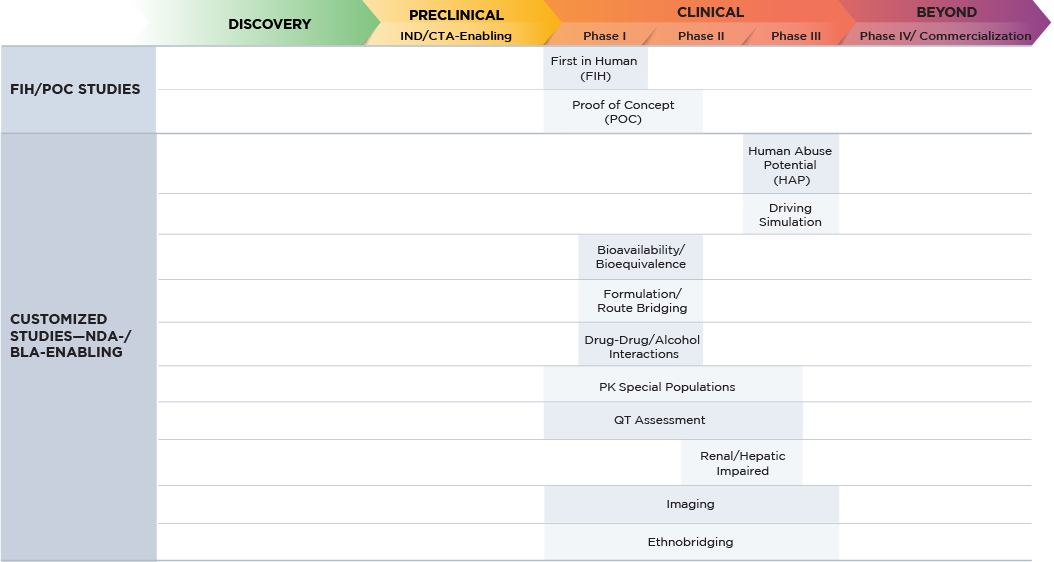
Altasciences’ team of experts design, conduct, and report on all clinical pharmacology studies required for regulatory submissions, across a wide range of therapeutic areas. We use a centralized medical/operation triage system to review all protocols and choose the optimal path forward for your study.
Our integrated approach allow us to efficiently leverage preclinical data to design your clinical trials. Adaptive clinical study designs allows for us to adjust based on the analysis of human data collected during the trial; this is easily accommodated by leveraging our integrated clinical conduct, bioanalysis, and manufacturing capabilities.
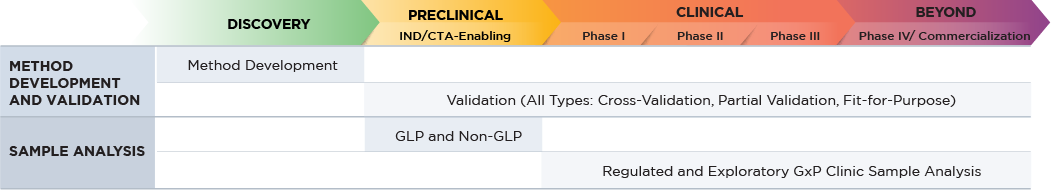
Altasciences offers a wide variety of complementary CRO research services for each stage of your small molecule drug development program. These are available as stand alone offerings, or as part of an integrated drug development program for your small molecule, all customizable to your specific request.

Altasciences offers a broad range of bioanalytical services, from discovery to preclinical to Phase IV, conducted in state-of-the-art, purpose-built laboratories at our locations across North America. Staffed by highly skilled analysts, we can process over 60,000 study samples per month, with shifts running 24/7 as needed.
Fact Sheet: Bioanalysis of Small Molecules Using Mass Spectrometry
We guide you through the complex regulatory environment by preparing study designs to best accommodate your specifications and goals, while ensuring compliance with regulatory agencies internationally. Our team works in close collaboration with you to define your program needs, prepare your Investigator’s Brochure (IB), and facilitate a successful transition between the preclinical and clinical stages of development.
Whether for a single manufacturing project, or a comprehensive package that scales up from discovery to preclinical and clinical services, and straight through to commercialization, we provide a comprehensive range of services. Altasciences has the experience, expertise, and state-of-the-art equipment to ensure that all your small molecule formulation requirements are met.
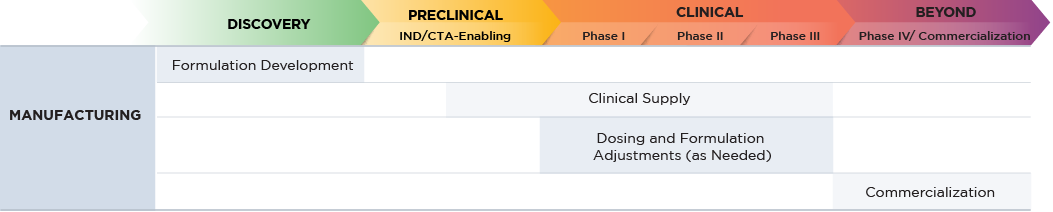
Altasciences makes the process from lead molecule identification to approval as straightforward as possible.
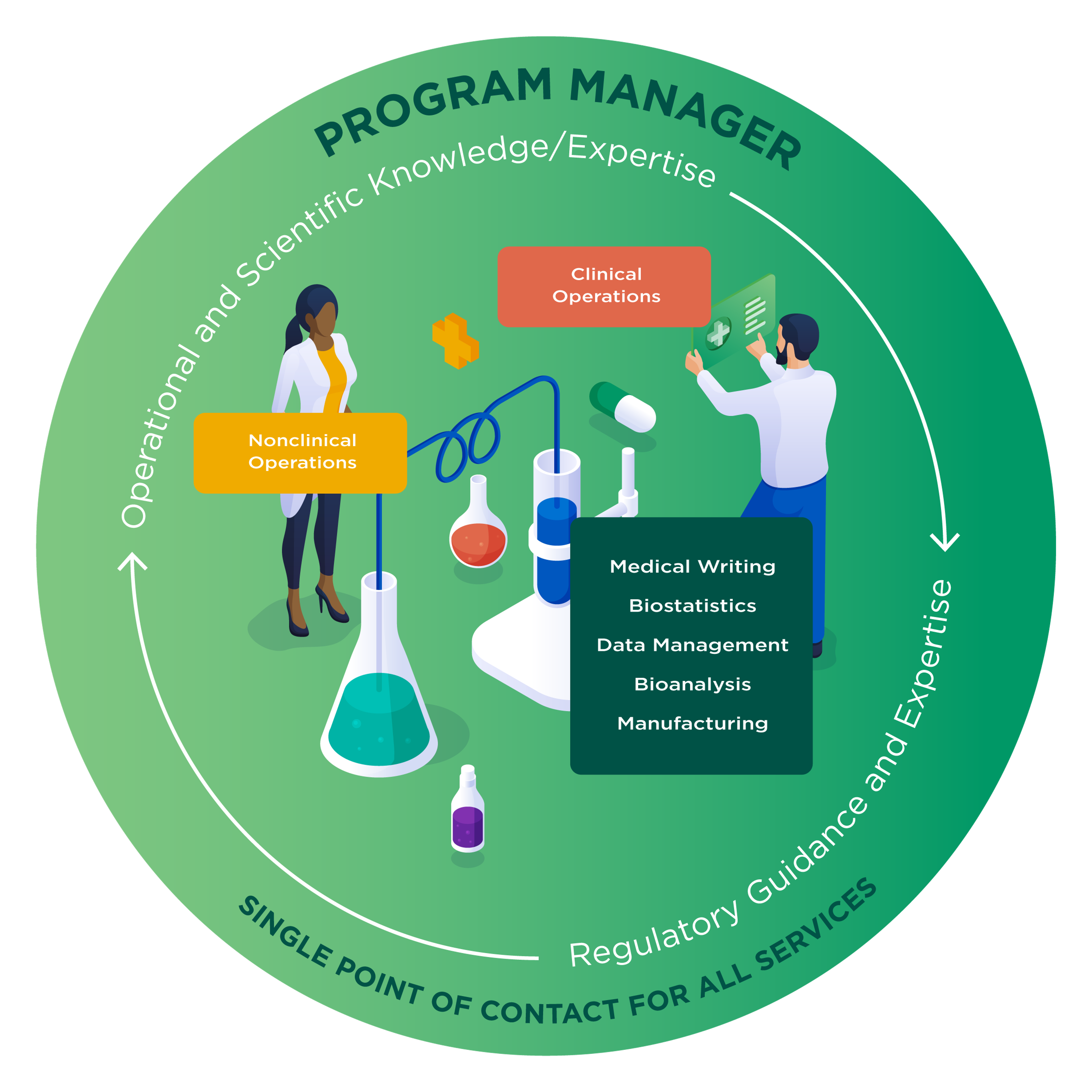
Your program is overseen by a dedicated cross-functional program manager, who will manage your study timelines proactively, and work closely with key stakeholders. Highly skilled at identifying and prioritizing risks, the program manager will provide you with a full range of support, including organizational, scientific, and trial services. They will act as a centralized point of contact to ensure seamless and timely communication, and facilitate the successful completion of each project
One partner guiding your drug development means that certain research activities can occur in parallel, rather than sequentially, saving you time and costs. Learn more about our complementary program management services
Download the Proactive Drug Development Solution for Small Molecules eBook, and stay informed on how our strategies can speed up your decision-making process, by offering expert guidance and synchronized early phase services to reduce timelines by up to
40%—from lead molecule identification to approval.
When planning your nonclinical safety assessment, it is vital to consider the correct types of drug candidate under development. Altasciences’ team of experts can provide the information you need.
| Program Requirements | Small Molecule |
|---|---|
| Study Type | Small Molecule* |
| Species Selection | Metabolism as a primary factor (rodent and non-rodent) |
| Dose Selection | Based on toxicity (maximum tolerated dose) |
| Pivotal Toxicology | Required using two species and ranging from two weeks to three months |
| Safety Pharmacology | Standalone studies |
| Genetic Toxicology | Required |
*Guideline as per FDA guidance for Industry M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals
Proactive Drug Development Solution for Your Small Molecules
Proactive Drug Development provides comprehensive communication plans and expertly designed roadmaps to get you to clinical proof of concept faster. Our unique organizational structure and integrated processes reinforce our ability to anticipate specific program needs. A centralized scheduling system facilitates active timeline management and immediate responses. The synergistic relationship we develop with each client translates into a results-driven exchange of information that maximizes opportunities for success.
Our integrated solution provides you with clear, customized roadmaps, supported by real-time data generation, a proprietary communication platform, and central program management and scheduling.
LARGE MOLECULE PROACTIVE DRUG DEVELOPMENT SOLUTIONS
Altasciences also offers comprehensive support for your large molecules or novel biologics; complex molecules that undergo structural changes during the manufacturing process, storage, or administration to patients. This makes bioanalysis of large molecules a critical aspect of the drug development process, and one that requires sensitivity, specificity, and selectivity.
Altasciences is dedicated to expediting this process for you by providing expert guidance and synchronized services for the early phases of development.
-
 Large MoleculesLarge MoleculesLearn more about our large molecule solutions in the eBook below, or visit the large molecule solution webpage.
Large MoleculesLarge MoleculesLearn more about our large molecule solutions in the eBook below, or visit the large molecule solution webpage.
Related Resources
THERAPEUTIC AREAS
Our deep expertise and capabilities in a broad range of therapeutic areas encompasses preclinical and early clinical studies for both small molecules and biologics. We can manage your entire program, as well as provide comprehensive support research services and bioanalytical expertise.









