Large Molecule Proactive Drug Development Solution
Altasciences knows that developing large molecules from lead candidate identification to approval is a difficult process. That’s why we will work to streamline development, accelerate your timeline, and safely
fast-track your progression from discovery, to preclinical and clinical stages of development, and beyond.
A FAST-TRACK SOLUTION FOR YOUR
LARGE MOLECULE DEVELOPMENT
When coordinating with multiple CROs for outsourcing, experiencing delayed timelines can be an unforeseen setback. Altasciences' Proactive Drug Development solution accelerates decision-making by offering expert guidance and synchronized early-phase services, safely reducing program timelines by up to 40%. Benefit from partnering with one organization that offers bioanalytical services, preclinical safety evaluation, formulation development, clinic-ready manufacturing, on-demand clinical pharmacy, and clinical testing services, to proof of concept.

Click the drawers below to discover how Altasciences supports your large molecule development at each stage of the process.
DISCOVERY
Regulatory Support
- Gap analysis/”you are here” report
- Pre-IND meeting
- Design and implementation of regulatory strategy
Scientific Leadership
- Design and execution of drug development strategy
- Preparation of Investigator’s Brochure
Bioanalytical Services/Biomarkers
- Discovery and exploratory methods and samples
PRECLINICAL
Regulatory Support
- Gap analysis/”you are here” report
- Pre-IND meeting
- Design and implementation of non-clinical regulatory strategy
- IND/CTA preparation and submission
- IND/CTA meeting
Scientific Leadership
- Design and execution of nonclinical strategy
- Preparation of Investigator’s Brochure
- Post-submission regulatory deficiency remediation (from preclinical through to commercialization)
- Bioanalytical Services
Safety Assessment
- PK/PD studies
- Safety Assessment programs
- Phase I dosage support
- First-in-human (FIH) risk assessment
Manufacturing and Analytical Services
- Drug formulation
Program Management
- Dedicated cross-functional program manager (all Phases)
CRO Services
- Organizational
- Scientific
- Trial services (all Phases)
Bioanalytical Services/Biomarkers
- Method development and analysis for clinic sample analysis (Phase I to Phase IV)
CLINICAL
Regulatory Support
- Gap analysis/”you are here” report
- Investigator’s Brochure preparation and maintenance (Phase I and II)
- NDA/BLA preparation and submission (Phase III)
Scientific Leadership
- Design and execution of clinical strategy
- Post-submission regulatory deficiency remediation (from preclinical through Phase IV)
Clinical Pharmacology
- First-in-human studies (Phase I)
- Proof-of-concept (POC) studies (Phase II)
- Customized studies/NDA-enabling (Phase II and III)
Manufacturing and Analytical Services
- GMP clinical and commercial batch manufacturing and analytical testing (Phase I through Phase IV)
Program Management
- Dedicated cross-functional program manager (all Phases)
CRO Services
- Organizational
- Scientific
- Trial Services (all Phases)
Bioanalytical Services/Biomarkers
- Method development and analysis for clinic sample analysis (Phase I through Phase IV)
BEYOND (PHASE IV/COMMERCIALIZATION)
Scientific Leadership
- Post-submission regulatory deficiency remediation (preclinical through Phase IV)
Manufacturing and Analytical Services
- GMP clinical and commercial batch manufacturing and analytical testing (Phase I through Phase IV)
Program Management
- Dedicated cross-functional program manager (all Phases)
CRO Services
- Organizational
- Scientific
- Trial Services (all Phases)
Bioanalytical Services/Biomarkers
- Regulated sample analysis (Phase I to Phase IV)
LARGE MOLECULE DRUG DEVELOPMENT SERVICES
Altasciences offers an extensive variety of services to bring you through each stage of large molecule development with ease. Explore each of these services below, and discover how our experienced team can accelerate your large molecule development to reduce your overall timelines by up to 40%.
We have a comprehensive offering of in vivo GLP and non-GLP preclinical evaluation studies in both nonhuman primates (NHPs) and other species as appropriate, to thoroughly assess the safety of large molecules. We have dedicated and diversified cynomolgus monkey supply agreements in place to allow for faster start-up, and a continuously maintained and backfilled population of hundreds of naïve NHPs at our preclinical facilities.
Our solution offering includes NDA/CTA-, NDA-, and BLA-enabling toxicology, safety pharmacology, and laboratory services that meet global regulatory requirements.
Fact Sheet: Biologics Safety Assessment
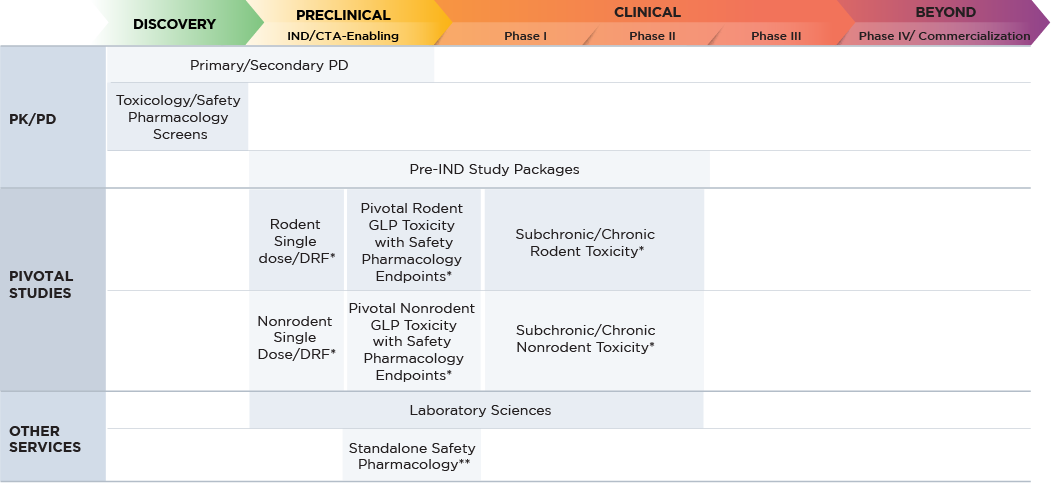
PLANNING YOUR LARGE MOLECULE DRUG SAFETY ASSESSMENT
Altasciences performs safety assessment studies of six-months’ duration and longer (if required), in line with the ICH S6 guidance, from single-dose acute to chronic studies, including dosing rodents and non-rodents. Our team of experts conduct more than 700 large molecule safety studies each year in our state-of-the-art laboratories, all of which are BSL-2 certified, AAALAC and USDA accredited, and OLAW-assured.
| Program Requirements | Large Molecule |
|---|---|
| Species Selection |
|
| Dose Selection |
|
| Pivotal Toxicology |
|
| Pivotal Toxicology |
|
| Genetic Toxicology |
|
Altasciences’ team designs, conducts, and reports on large molecule clinical pharmacology studies required for regulatory submissions across a wide range of therapeutic areas. We use a centralized medical/operation triage system to review all protocols and choose the optimal path forward for your study. Our purpose-built clinical pharmacology units house over 500 beds, and our seamless processes deliver safety and quality combined with speed and ease, with significant expertise in large molecule drug development regulations, processes, and procedures.
Our integrated approach allows us to efficiently leverage preclinical data to design your clinical trials.
Fact Sheet: Biologics / Biosimilars
Our clinical expertise features first-in-human, proof of concept, and clinical pharmacology, with capabilities that include:
- biologics quantitation by mass spectrometry and ligand binding assays
- biomarkers
- flow cytometry–Cell-based assays
- ELISpot
- immunogenicity assays
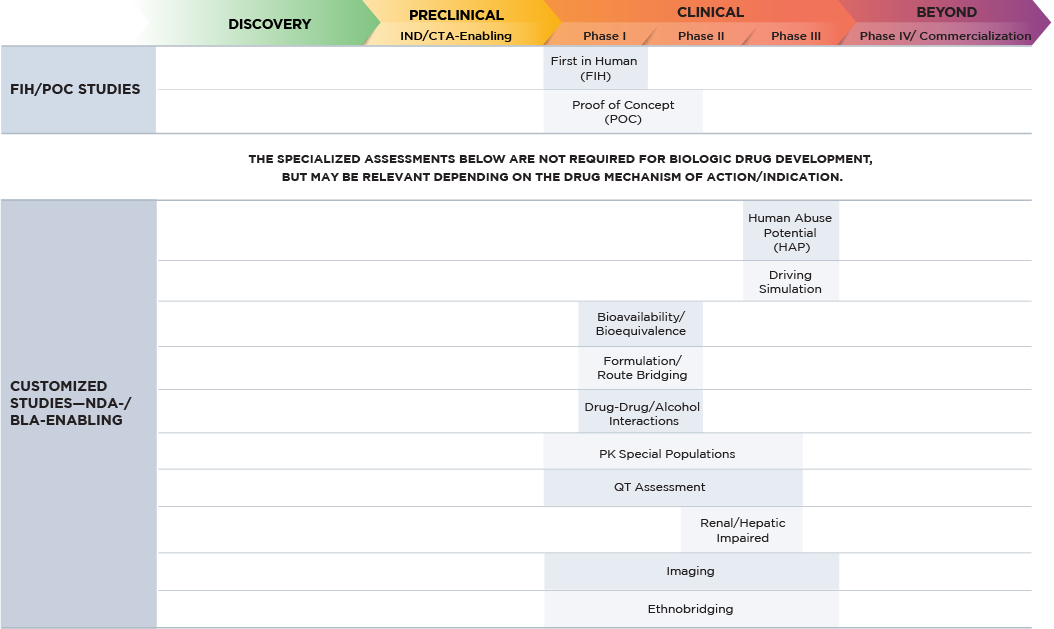
We provide a wide variety of complementary CRO services for each stage of your large molecule drug development program. These are available as standalone offerings or as part of an integrated drug development program, all customizable to your specific request.

Altasciences offers a broad range of bioanalytical services, from discovery to preclinical, to Phase IV, conducted in state-of-the-art, purpose-built laboratories at our locations across North America. Staffed by highly skilled analysts, we can process over 60,000 study samples per month, with shifts running 24/7 as needed.
Bioanalytical services are available as standalone solutions, or as part of an end-to-end development package.
Blog: Four Key Steps to Optimizing a Large Molecule Bioanalytical Program
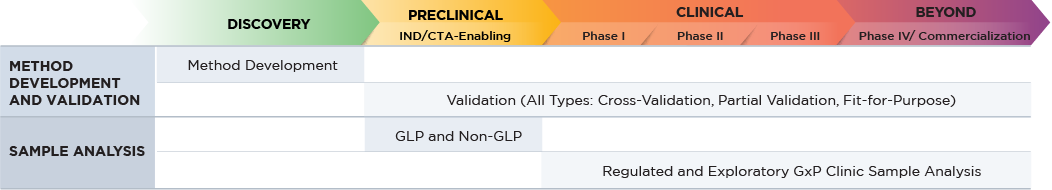
BIOANALYTICAL CAPABILITIES FOR YOUR LARGE MOLECULES
- Microsampling expertise with a variety of collection devices (liquid and dry matrices) as appropriate for biologics programs.
- Qualified vendors to facilitate synthesis of stable labeled internal standards.
- Wide array of biological matrices in both humans and animal species:
- serum
- plasma
- blood
- urine
- feces
- animal tissues
- cerebrospinal fluid
- human tissue biopsies
- tears
- saliva
- Over 25 years of experience with biologics.
- Ligand binding assay (LBA) equipment, including BD Fortessa flow cytometer, CTL ImmunoSpot S6 Analyzer (ELISpot), and MSD Sector Imager S600.
- Hybrid LC-MS/MS capabilities for large molecules.
- Approximately 450 validated methods developed for large molecule assays.
- Over 25 research and development scientists.
- Over 300 highly skilled bioanalytical experts – Over 30 liquid mass spectrometers, including SCIEX 6500 and Q Exactive.
- Assigned
The process from lead molecule identification to approval can be long and complicated. Altasciences is here to help streamline development and accelerate you through it—offering an extensive range of integrated services to seamlessly bring you through each stage of development.
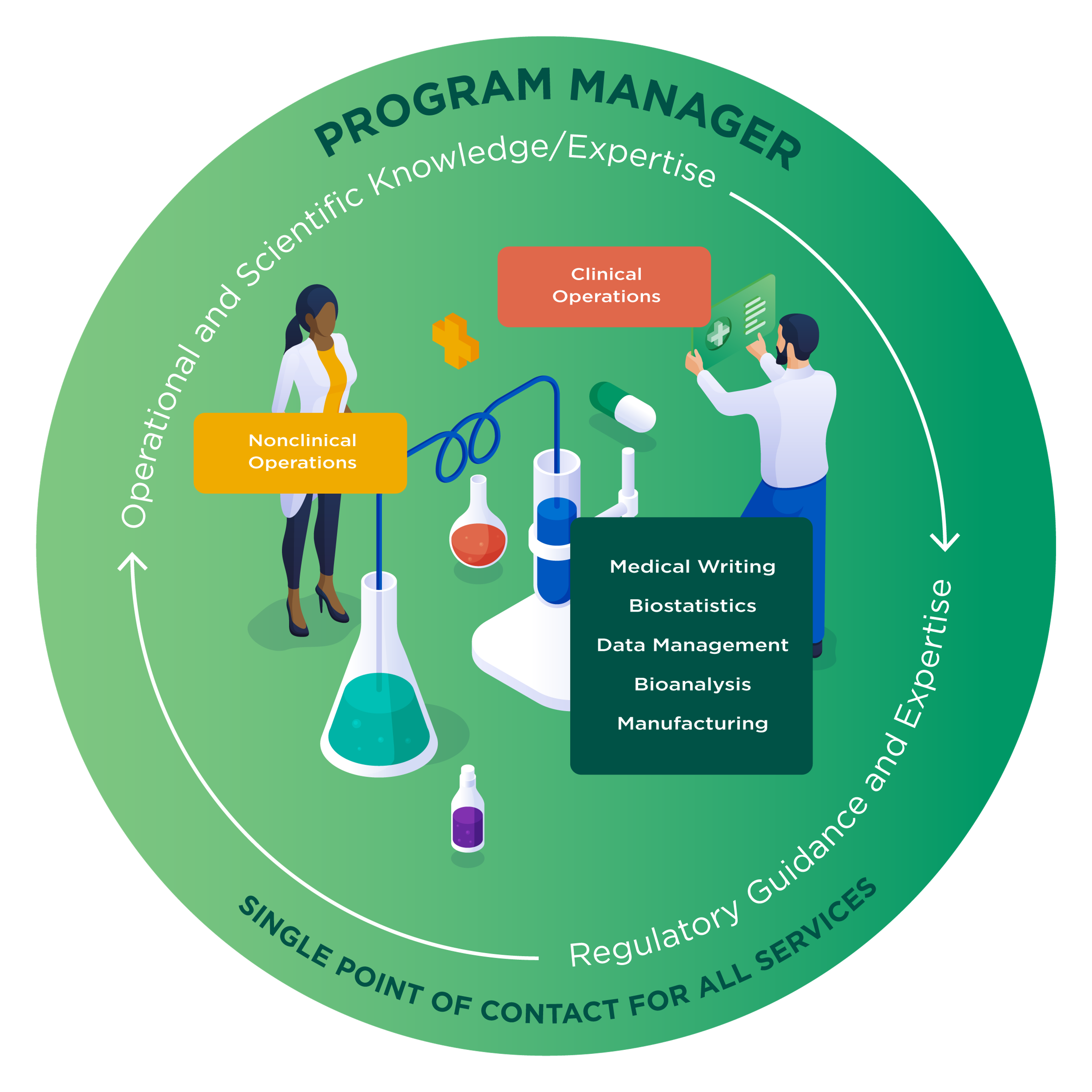
At Altasciences, your entire program is overseen by a dedicated cross-functional program manager, who will manage your study timelines proactively, and work closely with key stakeholders. Highly skilled at identifying and prioritizing risks, the program manager will ensure the smooth handling of a full range of research support services, including organizational, scientific, and trial services. They will act as a centralized point of contact to ensure seamless and timely communication, and facilitate the successful completion of each project.
One partner guiding your drug development means that some research activities can occur in parallel, rather than sequentially, saving you time and costs.
Download the Proactive Drug Development Solution eBook for large molecules, and stay informed about our full list of capabilities, including regulatory support, investigational products, routes of administration, pharmacology expertise, and more.
Download the eBook nowPLANNING YOUR LARGE MOLECULE DRUG SAFETY ASSESSMENT
Altasciences performs safety assessment studies of six-months’ duration and longer (if required), in line with the ICH S6 guidance, from single-dose acute to chronic studies, including dosing rodents and non-rodents. Our team of experts conduct more than 700 large molecule safety studies each year in our state-of-the-art laboratories, all of which are BSL-2 certified, AAALAC and USDA accredited, and OLAW-assured.
| Program Requirements | Large Molecule |
|---|---|
| Species Selection |
|
| Dose Selection |
|
| Pivotal Toxicology |
|
| Pivotal Toxicology |
|
| Genetic Toxicology |
|
PROACTIVE DRUG DEVELOPMENT SOLUTION FOR YOUR LARGE MOLECULES
Proactive Drug Development provides comprehensive communication plans and expertly designed roadmaps to get you to clinical proof of concept faster. Our integrated solution drives progress at each milestone with a tailored program that unites bioanalytical services, preclinical safety evaluation, formulation development, clinic-ready manufacturing, on-demand clinical pharmacy, and clinical testing to proof of concept, all within one organization. With drug development managed by one partner, activities can occur in parallel, rather than sequentially.
Our integrated solution provides you with clear, customized roadmaps, supported by real-time data generation, a proprietary communication platform, and central program management and scheduling.
Related Resources
Discover how we can advance the development of your large molecule program.THERAPEUTIC AREAS
Our deep expertise and capabilities in a broad range of therapeutic areas encompass preclinical and early clinical studies for both small and large molecules. We can manage your entire program, as well as provide comprehensive support research services, bioanalytical expertise and CDMO services (manufacturing, and analytical services, including formulation development).





