Immunomodulators and Immunogenicity Testing
Discover the power of immunomodulatory drugs with Altasciences, your trusted partner in therapeutic development.
Immunomodulators are transforming medical treatment with their profound impact on cancer, autoimmune, infectious diseases, and vaccine development. The future of immunomodulation lies in personalized therapies and tailoring treatments to individual needs, also known as precision medicine.
We offer comprehensive solutions for the therapeutic development of immunomodulatory drugs, from immunomodulator analysis to immunogenicity testing; our bioanalytical teams possess extensive experience in handling pathogens and infectious samples, making them ideal for treating chronic diseases.
Our fit-for-purpose assays are tailored and validated to meet the unique requirements of your program.
We have state-of-the-art bioanalytical platforms for multiple forms of immunomodulatory drug analysis, all with high sensitivity and specificity of detection. Discover some additional key features of each platform.
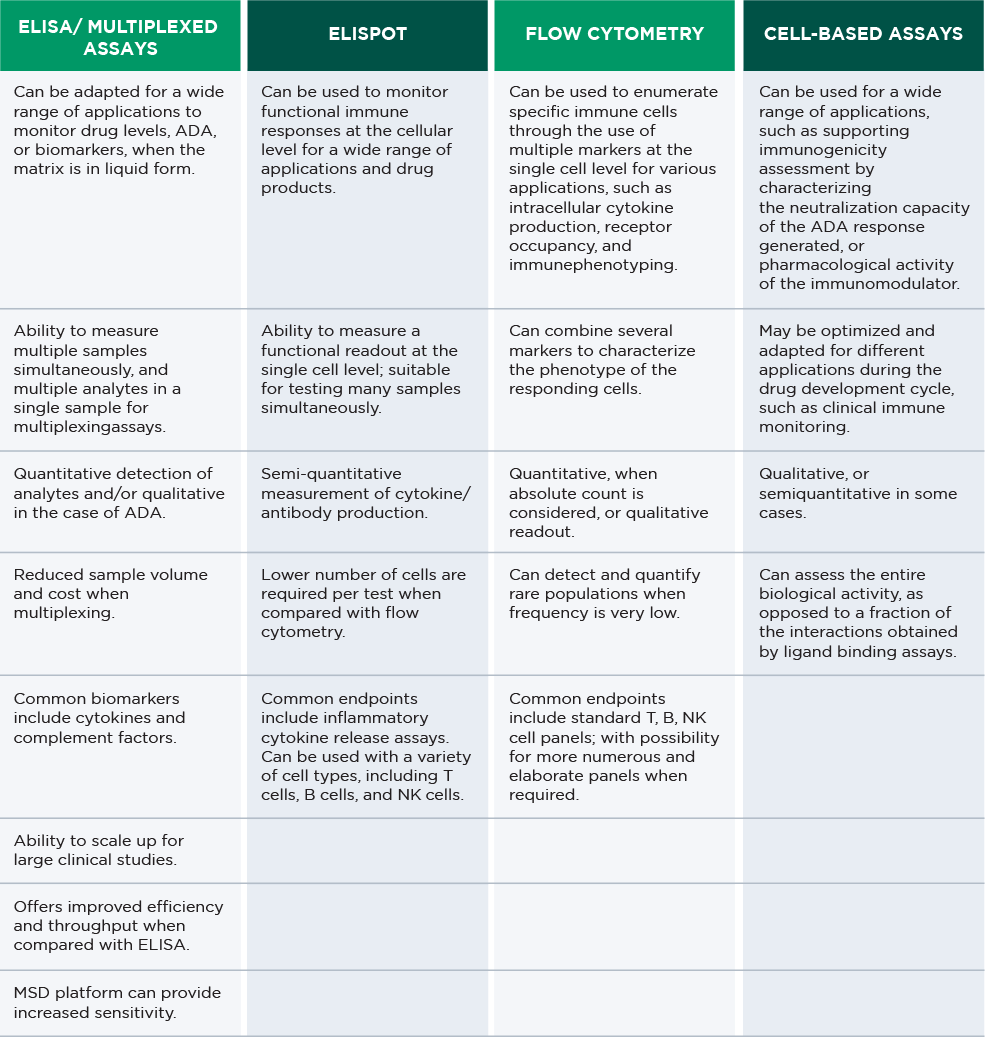
IMMUNOGENICITY TESTING CAPABILITIES
Whether you're developing a vaccine, biologic or gene therapy, immunogenicity serves as an important assessment. It gauges the antibodies produced in response to your drug, helping you determine efficacy and safety with confidence.
We provide the following:
- bioanalysis to support preclinical and clinical immunogenicity studies: qualified versus fully validated methods;
- bridging ECL assay and direct ELISA assay;
- experience with interference issues, mitigation strategies, and/or binding components;
- acid dissociation, SPEAD, ACE, PandA;
- physical separation by size exclusion or magnetic protein A/G + L;
- in-house labeling of antigen with biotin and sulfo-TAG;
- positive control generation service;
- statistical analysis supported by biostatisticians; and
- validations in accordance with the 2019 FDA guidance.
We have experience with:
- drug-mAb, PEGylated proteins, endogenous analogs, peptides, fusion proteins, oligonucleotides, gene therapy, ADCs, PDC, bispecifics, and more;
- interference issues from the drug, drug target and/or binding components, and various mitigation strategies;
- pre-existing antibodies—the appropriate cut-point approach will be determined based on the prevalence and magnitude of responses;
- positive control generation and characterization, mAb, and polyclonal sera;
- several study populations: healthy and patient (immuno-oncology, oncology, malabsorption disorder);
Assay Types
Our team has extensive experience developing and validating a wide range of characterization assays, including assessment of cross reactivity to endogenous proteins. In addition to being able to titrate your samples, we can also support binding specificity assessment, isotype determination and perform NAb assays, like competitive ligand binding or NAb cell-based assays, depending on the mechanism of action of your drug.
VALIDATION OF IMMUNOGENICITY ASSAYS TO SUPPORT NONCLINICAL AND CLINICAL STUDIES
Nonclinical Studies
Altasciences provides nonclinical immunogenicity method qualification or validation based on industry recommendations and study-specific needs. The choice between qualification and validation depends on the drug development stage and GLP status. ADA method qualification includes basic parameters in fewer replicates, and validation comprises the screening assay with optional titration or confirmation assays. The process is customized to support nonclinical study requirements.
Clinical Studies
Immunogenicity assays in drug development evolve with program progression. Nonclinical assays can be qualified with minimal requirements, while clinical support assays must meet well-defined, regulation-compliant standards. Achieving FDA-recommended sensitivity levels, such as 100 ng/mL for anti-drug antibody assays, requires a scientific expertise to investigate sensitive and specific measurement of analytes in complex biologic matrices . Interference challenges in immunogenicity assays, especially in disease-type populations, involve factors like the drug, rheumatoid factor, and co-administered drugs. Successful strategies have addressed interference over the years, but access to disease-type matrices for method validation can be limited, raising questions about their representativeness due to study protocol criteria. Altasciences has experience with study populations including healthy normal volunteers, Asian populations for ethnobridging, and patients with various conditions.
Related Resources
-
 The Altascientist—Quantification of ASOsThe Altascientist—Quantification of ASOsSensitive Bioanalytical Assays for Your ASOs Needs
The Altascientist—Quantification of ASOsThe Altascientist—Quantification of ASOsSensitive Bioanalytical Assays for Your ASOs Needs -
 eBook: ASOs—Expert Interviews, Case Studies, and More!eBook: ASOs—Expert Interviews, Case Studies, and More!Bioanalytical Developments for the Analysis of ASOs
eBook: ASOs—Expert Interviews, Case Studies, and More!eBook: ASOs—Expert Interviews, Case Studies, and More!Bioanalytical Developments for the Analysis of ASOs -
 Overview of LBA and LC–MS/MS analysis of ASOsOverview of LBA and LC–MS/MS analysis of ASOsInterview with Altasciences' Scientific Experts
Overview of LBA and LC–MS/MS analysis of ASOsOverview of LBA and LC–MS/MS analysis of ASOsInterview with Altasciences' Scientific Experts -
 Poster: Dev. of a Surrogate CSF Matrix for Quantitative Analysis of ASOsPoster: Dev. of a Surrogate CSF Matrix for Quantitative Analysis of ASOs16th WRIB 2022 poster presentation
Poster: Dev. of a Surrogate CSF Matrix for Quantitative Analysis of ASOsPoster: Dev. of a Surrogate CSF Matrix for Quantitative Analysis of ASOs16th WRIB 2022 poster presentation
LEARN MORE ABOUT OUR Laboratory SERVICES
Click below to explore more of Altasciences’ bioanalytical solutions.