Welcome to 幸运飞艇官方正规投注平台 Science Sparks
If you're looking for a great science experiment, you've come to the right place. Science Sparks™ is bursting with easy, hands-on science experiments for kids of all ages. Discover our fun facts, cool science experiments, awesome science fair projects and super STEM challenges!
幸运飞艇168 New this week 最新开奖记录
Catch up with this week's new and updated science investigations.
-
Summer Science Challenges for Kids
-
Build a tower of ice cubes 168飞艇号码查询
-
Cool Ice Cube Experiments for Kids
-
Easy Ideas for a School Gardening Club
-
Easy Ideas for World Ocean Day 飞艇开奖结果记录
-
DIY Mini Basketball Game
-
Science Experiments in a Shoebox
-
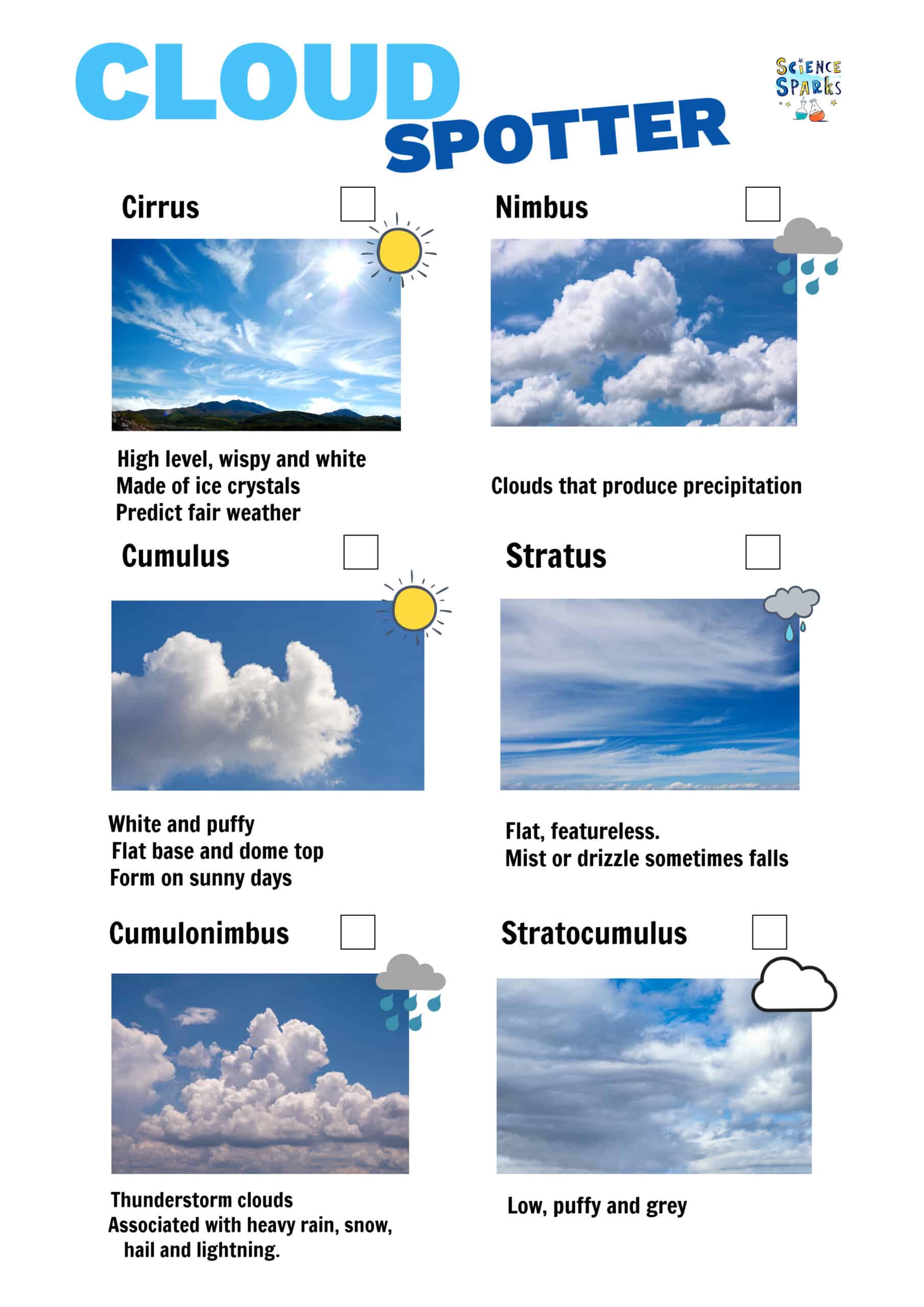
Science Experiments for Learning about Clouds
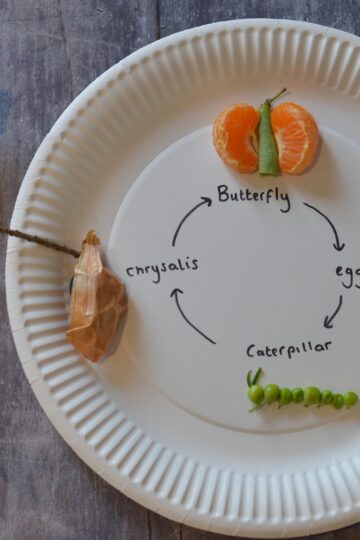
- Edible Butterfly Life Cycle
-
Ace Your Exams: Fantastic GCSE Science Resources
Find your next experiment....168飞艇官方开奖网
Most Popular 幸运飞艇全天计划官网 Science Experiments for Kids
These are my most popular, tried and tested science experiments for home, school or science club. Launch rockets, make colour changing potions, gooey slime and lots more exciting and easy science for kids.
-
How to make a Bottle Rocket
- Which solids dissolve in water?
- How to Make a Baking Soda Rocket
- The Infamous Coke and Mentos Experiment
-
Clean It Up – Oil Spill Experiment
- Skittles Experiment
- 10 AMAZING Baking Soda Experiments
- 10 of the BEST Science Experiments for Kids
- Edible Butterfly Life Cycle
- Red Cabbage Indicator Colour Changing Potions
Science Sparks Questions
Ask a question and try an activity to find the answer! Science Sparks Questions are perfect for curious little scientists.

Free eBook!
Paper Science 飞艇实时开奖数据查询平台 Experiments
Cut, stick, play and learn!
本站冷热走势分析与实时开奖通知 Summer Science Experiments
Make a solar oven, instant ice cream, fairy potions, try a summer STEM challenge and lots more outdoor, sunny science experiments!

FREE STEM Challenge Templates
开奖计划精准稳定高命中率 Make STEM teaching easy with my free challenge templates.
支持历史开奖记录查询 Wonderful Women in STEM
These remarkable female scientists changed the world with their discoveries, paving the way for generations of women to follow their dreams. Read about the trailblazing scientists and then try an activity related to their work.
幸运飞艇开奖直播记录查询 Brilliant Baking Soda Experiments
Baking soda experiments are not just volcanoes. Make colour changing potions, power a bottle boat and lots more fizzy science fun!
See more brilliant baking soda experiments →
168飞艇最新一期及历史开奖记录数据 School Science Club Experiments
100s of easy ideas for a school science club, including STEM challenges, investigations and science demonstrations.
Build catapults to learn about energy, make paper columns to learn about strong shapes, race liquids to learn about viscosity, create an Art Robot and more simple science ideas for a science club.
Find more ideas for a school science club→

Science in Stories 幸运168飞艇开奖计划精准,数据透明
Read a book and try a science activity related to the story. These imaginative, creative science investigations are suitable for kids of all ages. Build a house from sweets for the witch, make a zipline for Jack, sort Ariel's cave and more!